Advancing the Science
Currently, cord blood is approved for the treatment of 80 diseases. Additionally, the stem cells derived from umbilical cord blood and cord tissue have shown promising results in ongoing trials as investigational therapy to treat a wide range of other conditions. Each year, new therapies using stem cells from cord blood and cord tissue enter into clinical trials for the treatment of chronic and life-threatening diseases such as autism, cerebral palsy, multiple sclerosis, traumatic brain injury, stroke, and many others. With FDA-approval, these treatments can then be administered as a general practice.
Under a licensing agreement with Duke University, Cryo-Cell has been granted exclusive commercial rights to Duke’s intellectual property assets, FDA regulatory data, clinical expertise, and manufacturing protocols associated with various applications of cord blood and cord tissue stem cells. Through this agreement, Cryo-Cell intends to explore, test, and administer these treatments to patients with conditions for which there are limited FDA approved therapies.
Exciting Research Developments
In this video, Dr. Joanne Kurtzberg, Director of the Marcus Center for Cellular Cures at Duke University and Cryo-Cell’s Medical Director, offers an overview of the progress made in several clinical trials in which she is involved.
Since 2017, the Expanded Access Program operated under the Marcus Center for Cellular Cures at Duke University has opened the door for hundreds of patients to receive access to treatment for neurological conditions such as autism, cerebral palsy, stroke, and others using umbilical cord blood and cord tissue.
In addition, Dr. Kurtzberg reviews her work with the manufacturing of three types of therapeutic cells: cord blood, cord tissue mesenchymal stem cells (MSCs), and DUOC (cells manufactured from cord blood). These manufactured cells from the listed sources above are being explored in the treatment of conditions such as chronic brain injury, cerebral palsy, stroke, graft vs. host disease, autism spectrum disorder, COVID-acute respiratory disease (ARDS), hypoxic-ischemic encephalopathy, osteoarthritis, leukodystrophies, and multiple sclerosis.
The results of these clinical trials give us better insight into the direction of future research for patients in need of care. Cryo-Cell's vision is to move from Phase III clinical trials and Expanded Access to licensed biological products that will deliver approved therapies for a wide range of conditions.
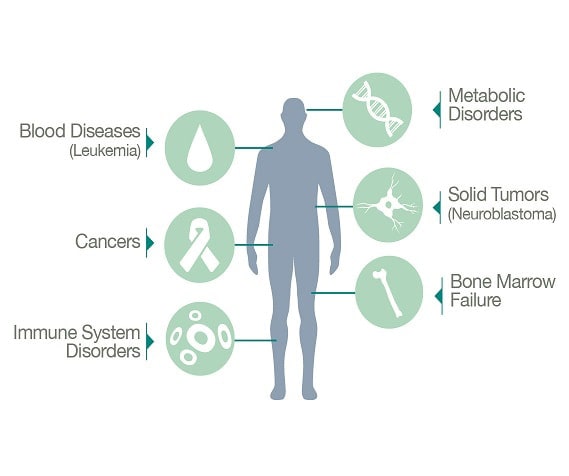 Currently, cord blood stem cells have been approved by the FDA in the treatment of nearly 80 diseases. In addition to these approved therapies, there are close to 350 clinical trials underway investigating the use of umbilical cord blood and umbilical cord tissue for stem cell transplantation, and this number promises to steadily increase. Cord blood stem cells are approved for numerous types of malignancies, anemias, inherited metabolic disorders and deficiencies of the immune system. The majority of cord blood transplants to date have been performed in patients younger than 18 years; however, advancements in regenerative medicine show promise for all ages. See all the diseases currently being treated.
Currently, cord blood stem cells have been approved by the FDA in the treatment of nearly 80 diseases. In addition to these approved therapies, there are close to 350 clinical trials underway investigating the use of umbilical cord blood and umbilical cord tissue for stem cell transplantation, and this number promises to steadily increase. Cord blood stem cells are approved for numerous types of malignancies, anemias, inherited metabolic disorders and deficiencies of the immune system. The majority of cord blood transplants to date have been performed in patients younger than 18 years; however, advancements in regenerative medicine show promise for all ages. See all the diseases currently being treated.
Current trials show promise for cord blood in the treatment of strokes, heart disease, diabetes and more. Umbilical cord–derived stem cells, meanwhile, are undergoing clinical trials for the treatment of multiple sclerosis, sports-related injuries, and various neurodegenerative diseases including ALS (also known as Lou Gehrig’s disease) and Alzheimer’s.
Through an exclusive licensing agreement, Dr. Joanne Kurtzberg and her scientific team at the Marcus Center for Cellular Cures at Duke University will be conducting ongoing clinical trials under an FDA-approved IND to treat patients for indications such as autism, cerebral palsy, and other brain injuries; with the intent of administering these treatments at a clinic location(s) managed by Cryo-Cell. We believe that this collaborative license agreement will provide greater opportunity for patients with unmet medical needs waiting for treatment.
Cord blood and cord tissue stem cells are currently undergoing clinical trials for a variety of diseases including ones once thought untreatable:
Read more about the benefits of cord blood and cord tissue.
Stem cells’ role is critical for regenerative medicine. A stem cell is a special type of cell because it is the basis for all the other cells in our bodies. Stem cells have the ability to develop into one of many different types of cells. This process of a stem cell becoming a specific type of cell like a skin cell, blood cell or bone cell is known as differentiation. The other unique ability of stem cells is to replicate quickly. Combined, these abilities can quickly replenish different types of cells, making stem cells a driving factor or major enhancement in the healing process.
Umbilical cord blood and tissue stem cells
Stem cells in the umbilical cord blood were first discovered in 1978. The stem cells found in cord blood give rise to all the other blood cells and are the foundation of our bodies’ immune system. More recently, scientists discovered a rich supply of a different type of stem cell in the cord tissue. These stem cells give rise to the tissues that comprise our nervous system, sensory organs, circulatory tissues, skin, bone, cartilage and more.
Read all about the power of stem cells.
Stay up on the latest stem cell developments with our stem cell news blog. Read about the newest trials that are underway, how current trials are faring and new ways that cord blood and tissue stem cells are being used in regenerative therapies. For doctors and researches, the Stem Cell Insider provides a more detailed look at the latest stem cell news and showcases the latest advancements in our products to help ensure stem cells preserved with us are viable and pure.