Multiple sclerosis can rob a person of essential functions and disrupt his or her quality of life. Although drugs can be used to treat some of its effects, many FDA-approved clinical trials have been and continue to look at stem cells to treat the condition. The use of stem cells like those in cord blood as a treatment for the disease has been showing great long-term promise, but wiping out the body's immune system, as performed in a stem cell transplantation, has also shown some associated risks.
Stem cells like those in cord tissue, on the other hand, may be a safer alternative while still providing long-term results. Clinical trials using those types of stem cells, however, have yet to undergo the same level of scrutiny.
Despite stem cell treatments not yet being approved as a standard therapy, there have been some reports and news articles about people afflicted with multiple sclerosis traveling abroad for the opportunity to undergo similar stem cell treatments—and experiencing significant improvements in their condition.
What is multiple sclerosis?
Multiple Sclerosis (MS) is a progressive disease that damages the central nervous system. It currently affects about 250,000 people to 300,000 people in the U.S. and 2.3 million people worldwide. It is an autoimmune condition where a person’s own immune system attacks the fatty coating that covers and protects nerves of the spine and brain. Symptoms of MS can range from mild to severe and may include numbness and tingling, loss of vision, chronic fatigue, balance and coordination problems and sometimes a decline in memory and thinking skills. Damage from the disease can be permanent and lead to disability such as paralysis.
Multiple sclerosis often forms between 20 and 40 years of age, occurs in three or four females for every male and, interestingly, is much more common in colder climates.
Because multiple sclerosis often forms later in life, most individuals do not have any stored for use in clinical treatments. Instead, researchers have been using similar stem cells found in the bone marrow and peripheral blood.
Cord Blood–Like Stem Cell Studies
In 2019, Dr. Richard Burt at Northwestern University published the results from a study looking at how transplantations using stem cells like those in cord blood compare with FDA-approved standards of care in people with multiple sclerosis who have yet to respond to medication.
The outcome followed interim results, which showed that a stem cell transplantation improved functions in 94% of participants compared with 40% who improved among the group that did not undergo a transplant. In addition, the group that underwent a stem cell transplant saw an overall average improvement in their Expanded Disability Status Scale scores compared with an overall average decline in the control group.
For 2017, researchers gathered and analyzed 15 studies published between 1995 and 2016 involving 764 people with various forms of multiple sclerosis who underwent transplantations using stem cells like those in cord blood, giving us our most expansive look at how these stem cells can be used to treat the condition. The analysis showed a significant benefit up to two years post-transplantation, with the progress of the disease stopping in 83% of participants. Researchers also noted, however, a transplant-related mortality rate of 2.1%. The studies with a greater number of people suffering from the relapsing–remitting form of MS showed a lower mortality rate and less disease progression. (With relapsing–remitting multiple sclerosis (RRMS), patients have points when their disease is active followed by periods when they do not experience any symptoms.)
The results of the metanalysis follow the kind of results researchers have been seeing in clinical trials: In 2017, researchers published the results of 281 patients who underwent stem cell transplants between 1995 and 2006. It noted that the disease stopped its progression in nearly half (46%) of patients for up to 16 years. Eight transplant-related deaths were reported.
Also in 2017, researchers published the results from a small nationwide study of 24 individuals with aggressive RRMS. In that trial, 95% went into remission for at least one year, with 69% experiencing no new disease activity for 5 years. All participants did experience "life-threatening adverse events" as an outcome of the transplantation.
Success Story
Jennifer Molson was diagnosed with multiple sclerosis in 1996 at the age of 21. In short time, she was dependent on her boyfriend for nearly every facet of her life such as showering and eating. After undergoing a stem cell transplantation, she saw a major recovery. She has since returned to work full time and takes part in rigorous activities like skiing, kayaking and swimming.
In 2016, Canadian researchers released results from a 13-year study of 24 patients with a poor multiple sclerosis prognosis who underwent a transplantation using stem cell like those in cord blood to treat the condition. The participants as a whole showed no relapses, no new lesions, and no increase in brain atrophy. In addition, 35% of patients showed improvements in their Expanded Disability Status Score. One patient did die from transplantation-related complications.
In 2015, results of a three-year trial showed incredible promise in the treatment of multiple sclerosis using cord blood: Researchers at Presbyterian–St. Luke’s Medical Center in Denver treated 24 patients with RRMS using a combination of high dose immunosuppressive therapy (HDIT) followed by a stem cell transplant using their own blood–derived stem cells. Dramatic improvements were seen in every area the trial sought to measure: neurologic disability and quality-of-life and functional scores. Patients experienced long-term disease remission after undergoing the transplant and the administration of high-dose immunosuppressive drugs.
For the three years following the treatment, more than 90 percent of patients did not experience disease progression and 86.3 percent did not have any periods of relapse. Additionally, patients in that time period did not develop any new lesions related to their disease.
Treatment was associated with few serious early complications or unexpected adverse events. Though a small number of patients did have side effects from the immunosuppressive drugs, they were no different from the side effects typically experienced by MS patients taking the drugs who haven’t undergone stem cell therapy.
Another preliminary study published in 2015 and conducted at Northwestern Medicine further corroborates the previous findings. The study, published in the Journal of the American Medical Association, included 145 patients with MS who underwent a stem cell transplantation.
This study found that the stem cell transplantation may reverse disability and improve quality of life for patients with RRMS.
“The stem cell therapy gets patients off lifelong treatments and gives them results that have never been seen before with this disease,” writes said Richard Burt, M.D., chief of Medicine-Immunotherapy and autoimmune Diseases at Northwestern Medicine.
Cord Tissue as a Promising Alternative
It was due to some of the risk found in their clinical trial that the researchers at Ottawa decided to investigate a different type of stem cell, mesenchymal stem cells, like those found in cord tissue, opposed to hematopoietic stem cells, like those in cord blood.
"Our experience with hematopoietic stem cell transplantation has been very encouraging, but this therapy has serious risks and it is only appropriate for a very small percentage of people with aggressive early MS," said Dr. Mark Freedman, who is leading the clinical trial.
"On the other hand, we really don't know what the effect of mesenchymal stem cell therapy will be in people with MS," he said. "It involves a different treatment approach that does not require the use of chemotherapy and therefore has fewer risks compared with hematopoietic stem cell transplantation."
The currently on-going clinical trial began in 2015 and wants to see if these stem cell like those in cord tissue can reduce inflammation and even help repair damage already caused by the disease in 20 patients.
Stateside, results from a clinical trial studying the plausibility of using cord tissue for the treatment of multiple sclerosis (MS) were released in 2018 and showed great promise in the treatment of the condition.
The study followed 20 people who had been afflicted with MS for an average of nearly 8 years and underwent an infusion of cord tissue stem cells to treat the condition. It found that subjects experienced an improvement in their symptoms one month after treatment, and those improvements were sustained for one year in many cases. In addition, no subjects showed disease progression or new lesions. One participant was able to walk with a walker after being dependent on a wheelchair, and another was able to walk freely after previously requiring a walker.
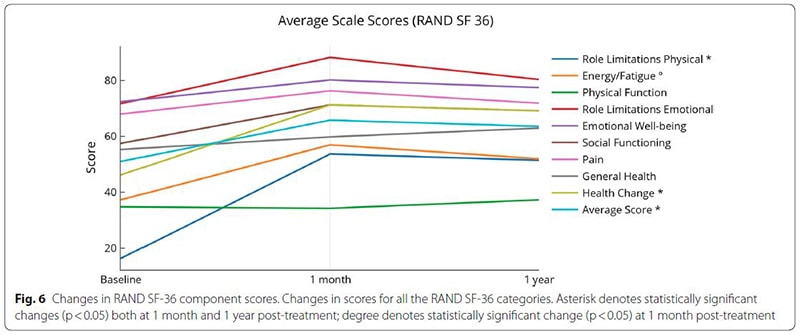 Biggest changes were in role limitations (both physical and emotional), energy and fatigue scores and overall health
Biggest changes were in role limitations (both physical and emotional), energy and fatigue scores and overall health
In conclusion, the study found that intravenous infusions of umbilical cord tissue stem cells over several days are not only safe but may hold benefits:
“This small study group saw improvement in bladder, bowel, and sexual dysfunction, walking, upper extremity physical function, energy and fatigue, general perspective of a positive health change and improved quality of life.”
This study follows a previous study that showed umbilical cord tissue helped restore balance to the immune system in people afflicted with multiple sclerosis.
How Stem Cells Treat Multiple Sclerosis
There are a few schools of thought on how cord blood and cord tissue can treat multiple sclerosis:
- The infused stem cells replace damaged tissue within the central nervous system.
- The transplanted stem cells replace the individual’s malfunctioning immune system.
- The infused stem cells mobilize local stem cells’ reparative and neuroprotective properties.
Current Clinical Trials
In addition to what may come now as Dr. Burt's phase II study has concluded, he is also conducting a phase III trial currently looking for patients with multiple sclerosis to undergo a stem cell transplant under one of two different regimens to help determine the most effective protocols.
A closed, active study is being conducted in the Netherlands and Scandanvia comparing the results of therapies using stem cells like those cord blood with the drug alemtuzumab in 100 patients.
A clinical trial out of the University of Jordan is looking for 60 participants with multiple sclerosis to study the effect of umbilical cord tissue stem cells and physical therapy on motor function.
Parents who have cord blood stored with Cryo-Cell and want to learn more about gaining access to these trials treating multiple sclerosis can use the link to make contact with us.