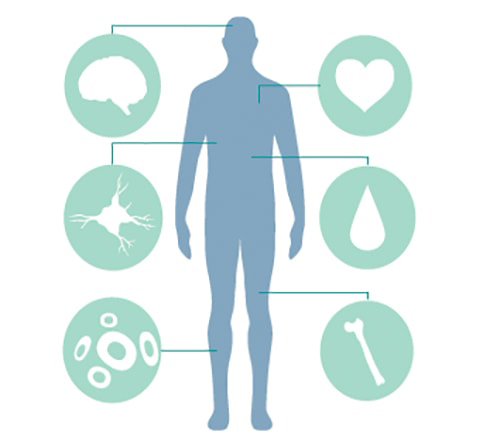
Stem cells are found throughout the body
The term stem cell may conjure up thoughts of some rare type of cell that can only be found in very specific locations. The opposite is really true. Stem cells are pretty ubiquitous in the body, appearing in many different organs and tissues including the brain, blood, bone marrow, muscle, skin, heart, and liver tissues. In these areas, they lie dormant until needed to regenerate lost or damaged tissue. They can do this because of their unique abilities to become many different types of cells and to replicate rapidly. (You can read more about the unique traits of stem cells here.)
As stem cells can be found throughout the body, it may seem as though they can easily be harvested for transplantation and regenerative medicine, but it’s the volume and the age of stem cells that are the main driving factors in where they are collected. Volume is important because there is no conclusive way to spur replication, so as of now, what you get is what you get. Age is also a factor because as stem cells age, they lose their ability to reproduce and differentiate into other cell types, they may become contaminated with a latent virus or affected by a disease, or they may have been exposed to toxins and have undergone mutation. They are also more likely to cause an autoimmune response, which is when your body attacks itself.
Embryonic
Found in large numbers during gestation, embryonic stem cells are by far the youngest stem cells and have the unique ability to become any type of cell in the body. There is a lot of controversy and ethical considerations concerning the embryonic stem cell. Thankfully, we can also acquire stem cells that form just a little bit later down the road and can be found in the umbilical cord blood and cord tissue. These stem cells are more limited in the types of cells they can become, something known as being tissue-specific, and they stay with us throughout our lives, which is why they are referred to as adult stem cells.
Cord Blood and Cord Tissue
Extracting the cord blood is painless and risk-free
The second to youngest stem cells are still called adult stem cells even though they can be collected at the time of delivery. Cord blood stem cells were discovered in 1978, and after the first cord blood transplant in 1988, the cord blood banking industry was formed. Cord tissue stem cells were discovered in the late '90s, and this discovery spurred cord tissue banking for many cord blood banks. Cord blood and cord tissue stem cells have the special quality of being the purest and youngest tissue-specific stem cells you can collect and function more quickly and effectively than adult stem cells from other sources. They are also easily collected at the time of birth. (Dive into the differences between cord blood and cord tissue.)
Placental tissue
Placental tissue can also be easily collected at the time of birth
The placenta and other amniotic tissues are also a rich source of the same type of stem cells found in cord tissue, and as with cord tissue, they can be easily collected at the time of birth. Despite these similarities to cord tissue, the major difference is that the placental tissue has a mix of the baby's and the mother's stem cells, and in order for these to be properly utilized in a stem cell treatment, they need to be separated. As the mother's stem cells often replicate more quickly than the fetal stem cells, placental stem cells are more likely to be preserved for the exclusive use of the mother.
Bone Marrow
A bone marrow draw requires the use of anesthesia and usually takes 20 days to fully recover
There are also areas where stem cells can be collected later in life. Bone marrow is rich in the blood-forming stem cells like those found in cord blood. To collect bone marrow stem cells, a needle is inserted into the soft center of the bone and requires the donor to undergo anesthesia. While it would be best to obtain bone marrow stem cells right from the person who needs them, the bone marrow procedure could be too much for the patient or the patient’s bone marrow could be too diseased. If this is the case, a matching donor must be found. A matching donor may be hard to obtain, and unfortunately, all non-related stem cell transplants come with a high degree of risk for an autoimmune response like graft-versus-host disease. (Read more about how cord blood and bone marrow compare.)
Peripheral blood
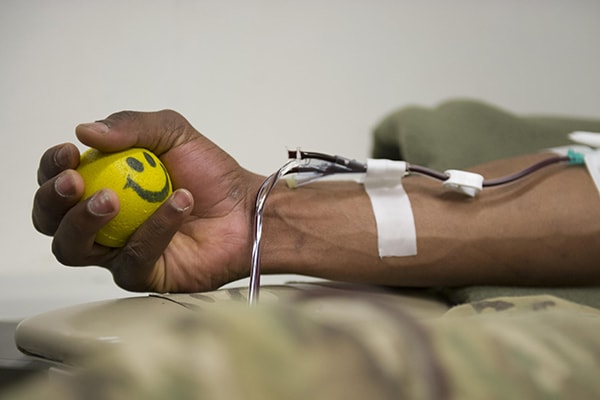
The procedure for capturing peripheral blood stem cells is like a long blood donation
As noted earlier, blood contains stem cells, just not too many. To gather a large number of stem cells from blood, the blood- and immune system–forming stem cells in bone marrow need to be coaxed out and collected. The non-surgical procedure is called apheresis. It begins days before the stem cell transplant with injections to get the stem cells in the bone marrow to enter the blood stream. The blood is then drawn from one arm and filtered through a machine to catch the stem cells from the peripheral blood. The rest of the blood is returned to the donor's other arm through another needle. Unfortunately, these stem cells have proven less effective compared with cord blood and bone marrow stem cells. In a meta-analysis of 9 trials totaling 1,111 patients, researchers found time to engraftment was slower and the frequency of graft-versus-host disease was greater in transplantations using peripheral blood than bone marrow. Researchers believe this has something to do with the removal of the stem cells from their bone marrow environment although the exact reason is not clear.
Fat (Adipose)-Derived
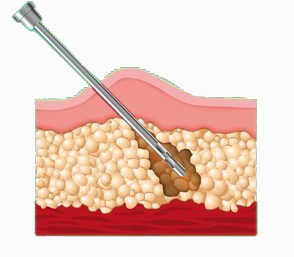
Obtaining adipose-derived stem cells requires a liposuction-like procedure that may itself take weeks of healing
Adipose stem cells are collected from fat tissue by way of an invasive liposuction-like procedure and are not the same as those found in cord blood or bone marrow. This means they are not used to treat the blood cancers and diseases that cord blood or bone marrow treat. The adipose tissue is more abundant in the same kind of stem cells found in cord tissue. These stem cells show promise for heart and kidney disease, ALS, wound healing and some autoimmune diseases.
Because stem cells taken from the patient and re-infused within 48 hours fall under different guidelines than stem cells collected through other methods, a market has sprung up for adipose stem cells, with many clinics touting their benefits in treatments that go well beyond current research. There is an inherit risk in using stem cell therapies neither approved by the FDA nor a part of an FDA-approved clinical trial.
Dental Pulp
Dental pulp can be collected as a child loses his or her baby teeth
A relatively new discovery is the stem cells in dental pulp. Teeth contain the same type of stem cells as adipose tissue and umbilical cord tissue, so once again, they are not used to treat the blood cancers and diseases that cord blood or bone marrow treat. Like cord tissue, however, dental pulp could hold future potential for heart and kidney disease, ALS, wound healing and some autoimmune diseases, and collection could involve simply saving all the teeth that fall out as the child grows.
It’s too early to know if dental pulp will prove to be an quality source of these types of stem cells, and the volume of stem cells is known to be small. As cord tissue stem cells are plentiful and have been being used in clinical trials for the past 20 years, comparing its progress to that of dental pulp is akin to comparing a great 9-year-old tee ball player to the upcoming major league superstar. Dental pulp as a source of stem cells is a new idea, and maybe it has potential, but it's still has to undergo years of trials, data collection and analysis before it will be a proven science.
Review
Stem cells can be found throughout the body, but the volume of the stem cells, the age and purity of the stem cells, the ease of collecting, the degree to which they have proven successful in transplants and clinical data and any ethical considerations are all major factors as to which is the preferred source. These are all factors where cord blood and cord tissue prove superior.