In brief: Ex vivo expansion has the potential to greatly increase the use of cord blood stem cells as a standard of disease treatment. A number of ex vivo expansion studies are in the second and third phases, suggesting that protocols are proving safe and feasible. Data show that neutrophil engraftment was significantly accelerated in the more recent studies, and in some cases, platelet recovery was also statistically improved.
Ex vivo expansion has the potential to greatly increase the use of cord blood stem cells as a standard treatment. A growing number of studies are looking at various methods of expanding cord blood stem cells, and a number of these studies are in the second and third phases, suggesting that ex vivo expansion protocols are proving safe, effective and feasible.
We previously told you a lot about UM171, which had been shown to greatly increase the number of cells from a cord blood unit. While UM171 is still on our list, other methods have also gained recognition. These expansion technologies are so promising that they have attracted small and big pharma, with investments and products from Novartis, Mesoblast, Gamida Cell, Magenta and Excellthera. A systematic review of current studies regarding cord blood–expanded products shows that time to neutrophil engraftment—a benchmark used to denote patient recovery—can also be reduced.
Expansion Types
Most of the ex vivo expansion studies thus far have involved the co-infusion of an expanded unit, or a part thereof, and a second unmanipulated unit, or fraction thereof.1 The evolution of expansion studies reflects the progression being made in our understanding of the topic. Early studies used cytokine combinations for expansion, but since 2008, the introduction of additional small molecules or feeder cell layers has been used to augment the effect of defined cytokine combinations. Specifically, the use of the following have been shown to either inhibit HSC differentiation or promote HSC self-renewal or accomplish both:
- Copper chelator
- Tetraethylenepentamine (TEPA)
- Notch ligand
- UM171
- StemReginin-1
- Nicotinamide (Nicord)
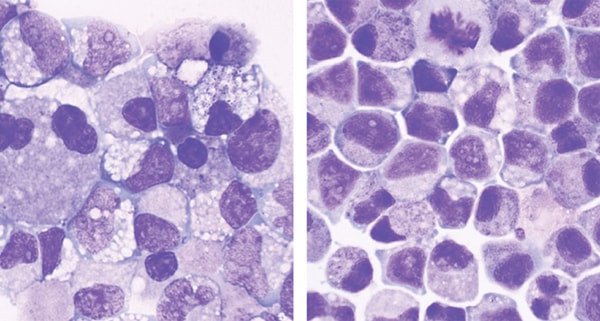 Stem cells extracted from cord blood and not treated with UM171 (left) appear less robust than similar cells treated with UM171 (right).
Stem cells extracted from cord blood and not treated with UM171 (left) appear less robust than similar cells treated with UM171 (right).More recent studies have also been using novel molecules such as HSC835 (Novartis) and MGTA456 (Magenta) or mesenchymal stromal cell (MSC) co-culturing, which is a promising strategy to mimic the physiological micro-environment within the marrow.
Findings
A number of individual studies observed that transplantation of expanded cord blood reduced the median days to neutrophil engraftment compared with historical double-cord transplant controls, with some studies reporting statistical significance in the time to neutrophil recovery. All studies that reported significant improvement in median neutrophil recovery rates vs controls used cytokines in combination with novel small molecules or with MSC co-culturing in the expansion protocol.
In some cases, platelet recovery was also statistically improved.
Results of larger randomized controlled trials are needed to understand the impact on patient outcomes and health-care costs.
Conclusion
It appears increasingly likely that expansion practices will be firmly established by the FDA and that researchers and doctors will have the ability to use just a portion of a collection to grow more stem cells. This would allow for the more widespread use of a single cord blood unit in adults or provide the opportunity for multiple treatments to be obtained from a single unit. Parents will then be more receptive to future clinical trials knowing that they will not have to use the entirety of their baby’s cord blood collection.3 This, in turn, can greatly expand the use of cord blood stem cells as a standard treatment.
|
Study Name
|
Study Number
|
Facility
|
Agent
|
Status*
|
|
Study of UM171-Expanded Cord Blood in Patients Who Need an Allogeneic Hematopoietic Stem Cell Transplant But Lack a Suitable Donor (Phase I–II)
|
NCT02668315
|
Maisonneuve–Rosemont Hospital
|
UM171
|
Recruiting
|
|
Study to Evaluate the Co-Infusion of Ex Vivo Expanded Cord Blood Cells With an Unmanipulated Cord Blood Unit in Patients for Hematologic Malignancies (Phase I)
|
NCT00343798
|
Fred Hutchinson Cancer Research Center
|
N/A
|
Completed
|
|
Infusion of Off-the-Shelf Ex Vivo–Expanded Cord Blood Progenitor Cells to Augment Single or Double Myeloablative Transplantation in Hematologic Malignancies (Phase II)
|
NCT01175785
|
Fred Hutchinson Cancer Research Center
|
N/A
|
Completed
|
|
Study of Single or Double Myeloablative Cord Blood With or Without Infusion of Off-The-Shelf Ex Vivo Expanded Cord Blood Cells in Hematologic Malignancies (Phase II)
|
NCT01690520
|
Fred Hutchinson Cancer Research Center
|
N/A
|
Recruiting
|
|
Study of Umbilical Cord Blood Cells Expanded With MPCs for Transplantation in Patients With Hematologic Malignancies (Phase III)
|
NCT01854567
|
Multi-National
|
Mesenchymal stem cells
|
Active
|
|
Allogeneic Stem Cell Transplantation of NiCord®, Umbilical Cord Blood-derived Ex Vivo Expanded Cells, in Hematological Malignancies (Phase I–II)
|
NCT01816230
|
Gamida Cell ltd
|
NiCord
|
Active
|
|
CordIn(TM), Umbilical Cord Blood-Derived Cells to Expedite Engraftment and Improve Transplant Outcome (Phase I–II)
|
NCT03173937
|
Gamida Cell ltd
|
CordIn
|
Recruiting
|
|
Study of StemEx®, to Treat Subjects With High Risk Hematologic Malignancies, Following Myeloablative Therapy (Phase II–III)
|
NCT00469729
|
Gamida Cell ltd
|
Stem-Ex
|
Completed
|
|
Allogeneic Stem Cell Transplantation of CordIn™, Umbilical Cord Blood-Derived and -Expanded Cells, in Hemoglobinopathies (Phase I–II)
|
NCT02504619
|
Gamida Cell ltd
|
CordIn
|
Recruiting
|
|
SAllogeneic Stem Cell Transplantation of NiCord®, Umbilical Cord Blood-Derived, Ex Vivo–Expanded Cells, in Combination With a Second, Unmanipulated Cord Blood Unit in Hemoglobinopathies (Phase I–II)
|
NCT01590628
|
Gamida Cell ltd
|
NiCord
|
Recruiting
|
|
Study to Evaluate Infusing HSC835 (LFU835-expanded Cells) in Hematological Malignancies (Phase I–II)
|
NCT01474681
|
Novartis
|
HSC835
|
Recruiting
|
|
Trial of Transplantation of NiCord®, Ex Vivo–Expanded, UCB-derived, Cells, and Unmanipulated UCB for Hematological Malignancies (Phase III)
|
NCT02730299
|
Gamida Cell ltd
|
NiCord
|
Recruiting
|
|
HSC835 in Patients With Hematological Malignancies Undergoing Single Umbilical Cord Blood Transplant (Phase II)
|
NCT01930162
|
Novartis
|
HSC835
|
Completed
|
|
Donor Cord Blood Cell Infusion Following Chemotherapy in Younger Patients With Relapsed or Refractory Acute Myeloid Leukemia (Phase I)
|
NCT01701323
|
Fred Hutchinson Cancer Research Center
|
Notch
|
Recruiting
|
|
Randomized Double Cord Blood Transplant Study (Phase II)
|
NCT00067002
|
Gamida Cell ltd
|
NiCord
|
Recruiting
|
REFERENCES
1. Science 2014; 345(6203):1509-12
2. Blood: 2013; 122(21)
3. J Clin Invest. 2014; 124(7):3121-8
4. Photo taken from http://www.sciencedaily.com/releases/2014/09/140918141155.htm