
The PrepaCyte-CB® is designed to specifically aggregate and sediment erythrocytic components, while retaining the white blood cells, including CD34+ hematopoietic stem / progenitor cells, which are recovered in the supernatant. It reduces up to 99% of the erythrocytes while retaining stem cells from human umbilical cord blood.2,3 An FDA 510K approved product, PrepaCyte-CB is manufactured under FDA current Good Manufacturing Practices (cGMP).
PrepaCyte CB is used in manual processing similar to Hetastarch, which allows for a nearly seamless transition in process operations, if replacing Hetastarch.
PrepaCyte CB reagent is packaged in a 500 ml bag of which a portion can be added directly to the Cord Blood Collection bag attached to a transfer/ freeze set (not included) to process and store. One 500 ml bag can be used to process up to eight cord blood units using 60 ml of PrepaCyte CB per cord blood unit. This media can be accessed by syringe through the syringe port, or a spike adapter can be added to the bag to supply the desired port.
How PrepaCyte-CB Compares with Other Processing Methods
PrepaCyte-CB is the most flexible cord blood–processing method, and it offers a number of special benefits and advantages over other processing methods. The end result to the following features of PrepaCyte-CB is a reduction in the time to neutrophil engraftment:
- PrepaCyte-CB has been shown to yield the highest number of colony-forming units (CFUs). The number of CFUs is currently the most accurate measurement to determine stem cell potency and the potential for stem cell engraftment.1,2,3
- PrepaCyte-CB has been shown to recover the greatest percentage of CD34+ stem cells from human umbilical cord blood.
- PrepaCyte-CB is the only processing type where TNC and CD 34+ recovery is not affected by the initial volume of the collected unit.
- PrepaCyte-CB has also been shown to provide the greatest reduction in red blood cell (RBC) contamination compared with all other processing methods.
- PrepaCyte-CB–processed samples have a significantly greater clonogenic potential than all current methods.
| |
PrepaCyte |
Hespan |
SEPAX |
| CFU Recovery % Post Thaw4 |
80.2 |
52.9 |
62.7 |
| RBC Depletion %4,5,6 |
99 |
82 |
84.7 |
Better Treatment Outcomes
(Click to view St. Louis post-transplant assessment in a new window)
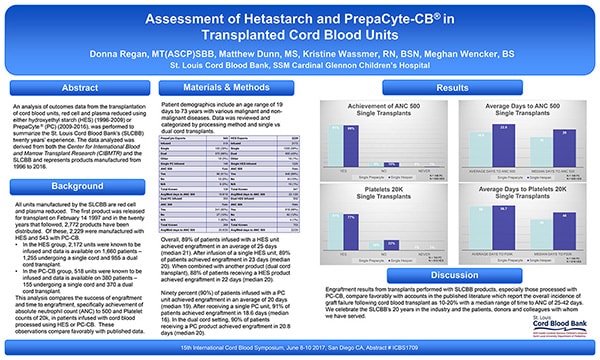
The result of the differences between PrepaCyte-CB and other processing methods has been shown to lead to better treatment outcomes, with PrepaCyte-CB–processed cord blood units engrafting more quickly than any other methods. In an evaluation of the probability of and the time to patient neutrophil recovery by the St. Louis Cord Blood Bank, PrepaCyte-CB had a median time of 16 days compared with 20 days for the Hespan group.7
"After infusion of a single HES unit, 89% of patients achieved engraftment in 23 days (median 20). … After receiving a single PC (PrepaCyte-CB) unit, 91% of patients achieved engraftment in 18.6 days (median 16)."
Additionally, when the data on engraftment times from a number of public data sources were compared, PrepaCyte-CB was found to engraft more quickly than all other cord blood–processing methods.
| Processing Method |
Days to Recovery* |
Public Data Source |
| Prepacyte-CB7 |
16 |
St. Louis Cord Blood Bank |
| HES7 |
20 |
St. Louis Cord Blood Bank |
| AutoXpress® (AXP)8 |
20 |
New York Cord Blood Center |
| Sepax9 |
21 |
Duke University Medical Center |
| HES/Sepax10 |
21.5 |
Bloodworks |
| HES/Sepax11 |
25 |
ClinImmune LabsA |
| *Recovery time is the median time to reach an absolute neutrophil count (ANC) of 500. |
Benefits for the Laboratory
PrepaCyte-CB is intuitive and easy-to-use, permitting rapid implementation within new and existing cord blood banks. PrepaCyte-CB allows batch processing and reduces "hands on" staff time. A number of cord blood banks have realized a time-savings of up to 50 minutes per cord blood unit using PrepaCyte-CB compared with the hetastarch processing method. PrepaCyte-CB also requires no costly capital equipment or maintenance fees. Only a standard laboratory centrifuge is required to concentrate desired cells after separation.
Instructions
Contact us for more details today!
Caution: Federal Law restricts this device to sale by or on the order of a licensed practitioner